Medicine lifecycle
Once a new medicine is no longer covered by a patent it can be manufactured by any company around the world.
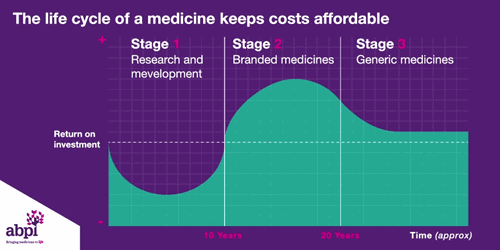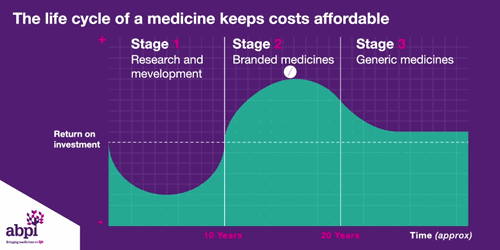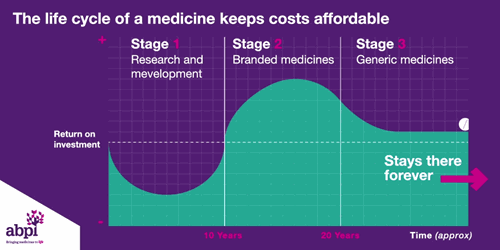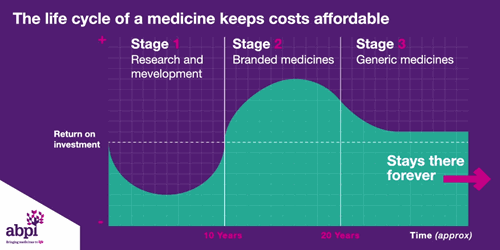
We typically think of three major phases of a medicine’s lifecycle.
The first is research and development which is where companies put in hundreds of billions of pounds to develop and test new medicines.
The second phase is when a medicine finally gets a licence, that company has exclusive rights to sell that medicine for a period of time, as long as the price is deemed to be appropriate by bodies like NICE.
After that period of exclusive rights which typically last about 10 years is the third and final phase of a medicine’s lifecycle and when a medicine reaches that stage, it stays there forever.
This is why a month's supply of cholesterol treatment today cost less than the price of a cup of coffee.
So, these three phases of the lifecycle of medicines are incredibly important because it allows companies to invest in medicines of the future, but it also makes sure that in the long run medicines become very affordable.
When the medicines lifecycle works appropriately, for every medicine that comes onto the market and creates new costs for the NHS, older medicines are losing their patent exclusivity and becoming much, much cheaper.
So, the medicines lifecycle working well helps keep spend on medicines under control.
What is the life cycle of a medicine?
The life cycle of a medicine keeps costs affordable
There are typically three important phases of a medicine’s life cycle. The first is research and development, where industry puts in hundreds of billions of pounds into developing and testing new medicines.
Once a medicine is licensed, a company has exclusive rights to sell that medicine to the NHS for a period of time at a price which has been approved by NICE.
The medicine is scrutinised for both the benefit it brings to patients and the its cost effectiveness for the NHS by a body called the National Institute of Health and Care Excellence (NICE).
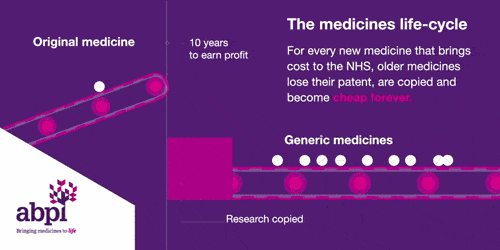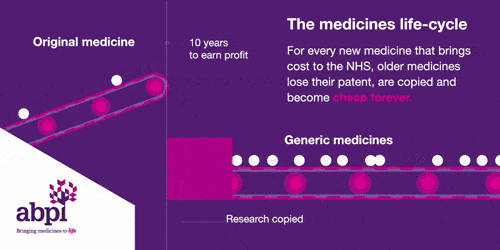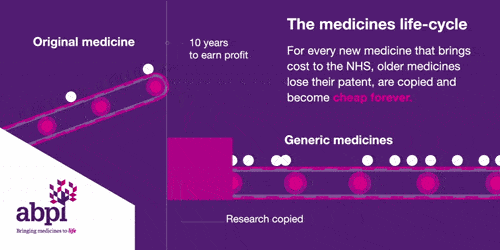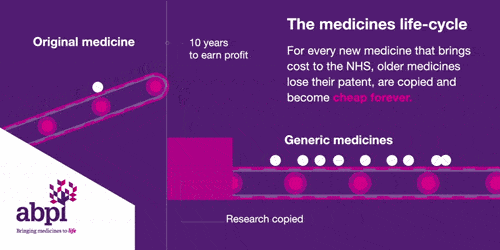
The medicines life-cycle
The final stage is where a medicine comes off patent and can be copied. At this point, due to extra competition in the market, the price is likely to drop significantly.
This is why major breakthrough medicines, like statins, now cost less than a cup of coffee, or why some cancer drugs cost just a few pounds per day.
When pricing a medicine, a company will take into account its research and development, the need to earn profits and create further finance which can be ploughed back in to future research for new medicines and vaccines.
This will include recouping the costs of the many medicines and vaccines which are likely to have failed along the development pathway.
Globally, pharmaceutical companies put around £140 billion a year in to researching and developing new medicines.
Last modified: 20 September 2023
Last reviewed: 20 September 2023