The case for agreeing medicine quality checks between the UK and EU
When medicines are made, they have to be checked by the manufacturers for safety and quality in a process known as batch testing. Countries with similar high standards and regulatory checks often agree a “mutual recognition agreement” (MRA) on these batch tests, so that companies don’t have to repeat the testing when a medicine crosses a border.
Currently, medicines exported from the UK to the European Union (EU) must be retested when they cross the border and enter the EU market – while the UK accepts imports from the EU without requiring duplication of such tests. This extra step, required by the EU, complicates the supply chain for medicines, adds unnecessary burden and costs and can delay medicines reaching EU patients by an average of six weeks[i], which has to be accommodated within delivery scheduling.
Under the EU-UK trade deal, both sides agreed to recognise each other’s site inspections of manufacturing site, but the deal did not go as far as agreeing to mutually recognise batch tests.
As both the UK and EU have such batch testing agreements with the same markets around the world – it is therefore arbitrary that the UK and EU do not have one.
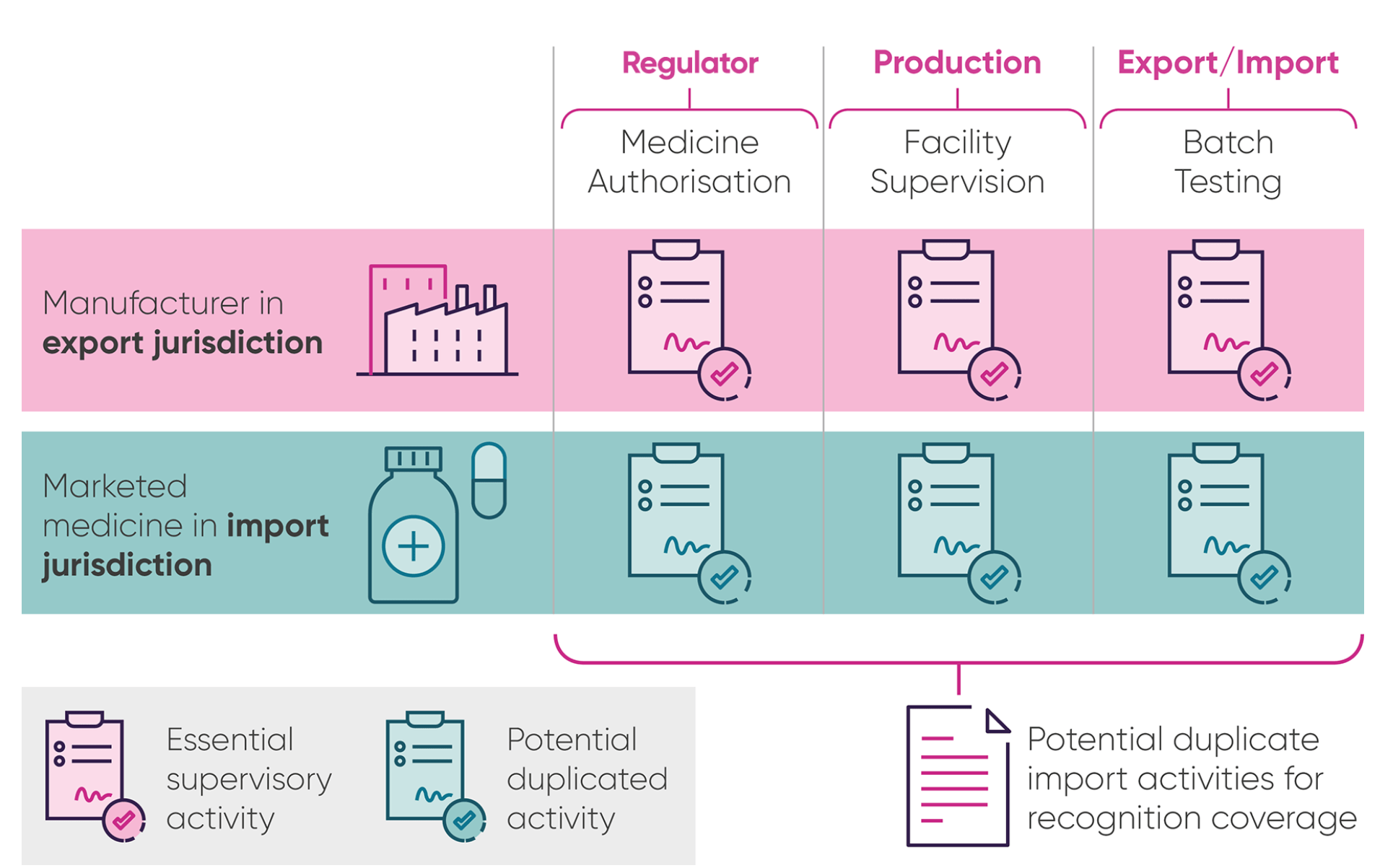
Figure. The process for moving medicines across the border and the role of National Competent Authorities (NCAs) such as national regulators like the MHRA
Download a hi-res version of the infographic.
| EU has MRAs with... | UK has MRAs with... |
|---|---|
| Australia | Australia |
| Canada | Canada |
| Israel | Israel |
| Japan | Japan |
| New Zealand | New Zealand |
| Switzerland | Switzerland |
| USA | USA |
Table: MRAs in place with the EU and the UK.
The benefits of a mutual recognition agreement on batch testing
Duplication of batch testing on exports from the UK to the EU has introduced a trade barrier for medicines supply, if both sides could agree to remove it would strengthen the supply chain resilience for medicines in Europe, and support the UK to become a more attractive destination for manufacturing investment by:
- Saving time and money: almost half (46%) of all medicines and pharmaceutical products exported from the UK go to the EU.[ii] Reducing the cost of duplicating a batch test on a product exported from the UK into the EU could save businesses £1,500 per batch. i
- Supply chain security: both the UK and EU have discussed the importance of safeguarding supply for critical goods such as medicines. They both have strategies which include working with international partners to find agreements that would make supply chains more resilient – this is one such area they should be prioritising.
- Attracting investment into Europe: since the UK already accepts EU batch testing without the need for repeated testing, agreeing to do the same for UK batch testing going to the EU would remove another cost/benefit consideration for future investment.
Last modified: 27 November 2024
Last reviewed: 27 November 2024